CRC News: June 30, 2020
Dear NCCRT Members and 80% Partners,
We hope you and your families are staying safe and well. We have several updates to share with you this week.
NCCRT Releases New Playbook on CRC Screening During the COVID-19 Pandemic
This just released resource from the NCCRT provides an action-oriented playbook to be adopted throughout the COVID-19 pandemic and aims to align NCCRT members, 80% pledged partners, and colorectal cancer screening advocates across the nation to work together to reignite our screening efforts appropriately, safely, and equally for all communities.
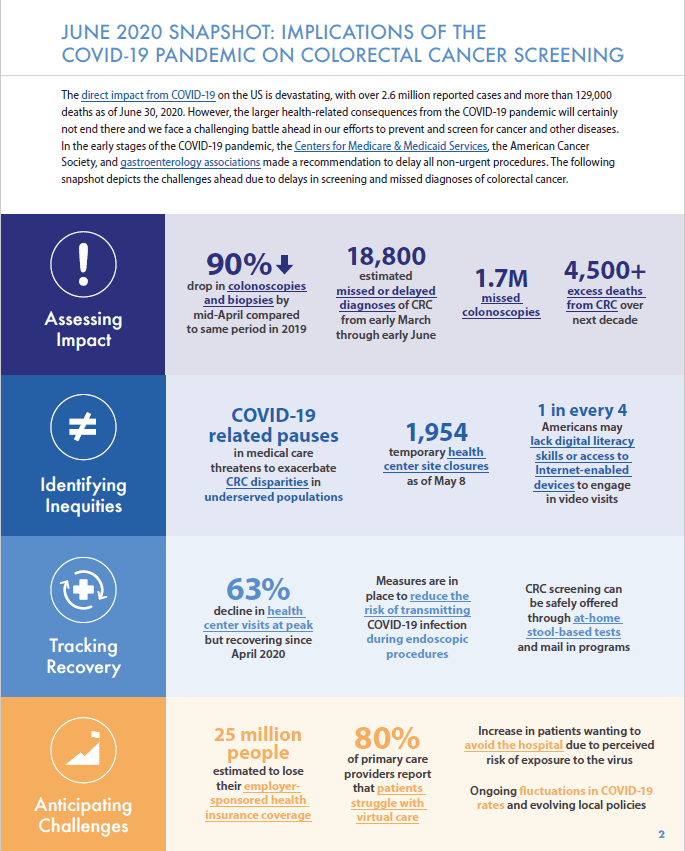
The COVID-19 pandemic has challenged efforts to address inadequate screening and inequities in colorectal cancer outcomes, hindering the progress toward our 80% in Every Community goals. In the early stages of the COVID-19 pandemic, leading agencies, such as the Centers for Medicare & Medicaid Services (CMS) and the American Cancer Society, made recommendations to delay all non-urgent procedures. Colonoscopies to detect colorectal cancer have been delayed or cancelled and patient fears about contracting COVID-19 have led to further reductions in screening. This drop has raised concern that COVID-19 related screening delays will lead to missed and advanced stage colorectal cancer diagnoses and to excess deaths from colorectal cancer. Moreover, this burden will likely not be evenly distributed as screening disparities may be exacerbated in communities and populations that are disadvantaged by both old and new challenges in the COVID-19 era.
The colorectal cancer fighting community stands prepared and well-positioned to respond to and overcome the difficult task ahead, and this document offers the latest data, research, and clinical guidelines available. A special note of recognition to our playbook authors: Durado Brooks, Rachel Issaka, Steven Itzkowitz, Michael Sapienza, Ma Somsouk, and Richard Wender.
New Summary Report on Links of Care Pilot to Increase CRC Screening for Underserved Patients
The newly released report, Report on a Pilot Project to Increase Colorectal Cancer Screening Rates and Ensure Access to Specialty Care for Underserved Patients, provides an overview of the Links of Care pilot project (2015-2017), which implemented evidence-based strategies to increase screening rates and timely access to specialists after abnormal screenings in three Federally Qualified Health Centers (FQHCs). Participating FQHCs successfully increased CRC screening rates by 8-28 percentage points, secured low- or no-cost colonoscopies from specialty care providers, and implemented patient navigation to ensure timely follow-up to diagnostic services. The report outlines key facilitators to success. Congratulations to the evaluators and authors that contributed to this publication, Lesley Watson, Kara Riehman, Mary Doroshenk, Rentonia Williams, Vonda Evans, Lynn Basilio, Maryanne Goss, and Roshan Paudel, as well as the numerous individuals that contributed to the pilot projects’ success.
New Mailed FIT Publication
In June 2019, the Centers for Disease Control and Prevention (CDC) convened a meeting of subject matter experts and stakeholders to answer key questions regarding mailed (fecal immunochemical test) FIT implementation in the United States. The recently released publication, Mailed fecal immunochemical test outreach for colorectal cancer screening: Summary of a Centers for Disease Control and Prevention–sponsored summit, summarizes points of agreement and includes practical recommendations for implementation. Report authors include numerous NCCRT members and two NCCRT Steering Committee members, Gloria Coronado and Lisa Richardson.
National Comprehensive Cancer Network (NCCN) Issues New CRC Screening Guidelines
On June 8th, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) issued Version 2.2020 of the Colorectal Cancer Screening guidelines. Access for registered users is available on the NCCN website.
Reminder: Request for Proposals to Update NCCRT’s Steps Guide to CRC Screening for Community Health Centers
The NCCRT is seeking proposals to update, revise, and modernize the signature NCCRT resource, Steps for Increasing Colorectal Cancer Screening Rates: A Manual for Community Health Centers, originally published in 2014. The Steps Guide provides succinct steps-by-step instructions for community health center teams for implementing screening programs and policies along with guidance on evaluation and outcome tracking. We are now seeking a contractor to update the guide to reflect current evidence-based best practices, include recent case examples, expand relevancy to all primary care settings, and to include a “how to” brief on timely follow up to abnormal stool-based test results. The deadline for proposals is July 18th, 2020.
Please email Caleb Levell, NCCRT co-director, ([email protected]) with any questions related to the RFP. We also welcome your suggestions for potential vendors. Email notification of intent to apply and questions regarding the proposal are due July 8th, 2020. All questions and answers will be posted to the RFP webpage.
Many thanks for the great work you do.
The NCCRT Team
Did you miss a past edition of CRC News? Visit the News & Research archive.
Do you have a colleague that would like to be adding to receive our newsletter? Please encourage them to sign up to receive CRC News.[vc_tweetmeme][vc_facebook]
We Highlight Successes, Leaders, Best Practices, And Tools That Are Making An Impact In The Nationwide Movement To Reach 80% Screened For Colorectal Cancer.
Do you have a suggestion for a future blog topic? We welcome you to share your suggestions by emailing [email protected].
Blog Policy
Opinions expressed in these blog posts are that of the author and do not represent policies of the National Colorectal Cancer Roundtable or the author’s institution.
Our staff moderate all comments on the 80% Blog. While we do not censor based on point of view, we will delete or edit comments that are offensive or off topic. Click here to view full version.
© 2024 American Cancer Society National Colorectal Cancer Roundtable. All rights reserved.


