A Playbook for Reigniting Colorectal Cancer Screening as Communities Respond to the COVID-19 Pandemic
Reigniting Colorectal Cancer Screening as Communities Face and Respond to the COVID-19 Pandemic: A Playbook
This resource provides an action-oriented playbook to be adopted throughout the COVID-19 pandemic and aims to align NCCRT members, 80% pledged partners, and colorectal cancer screening advocates across the nation to work together to reignite our screening efforts appropriately, safely, and equally for all communities.
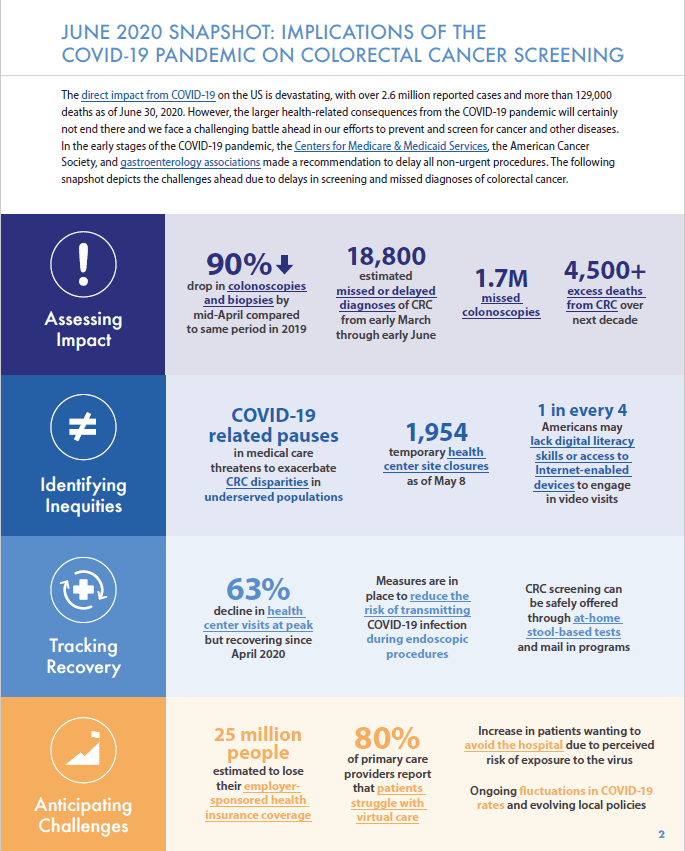
The COVID-19 pandemic has challenged efforts to address inadequate screening and inequities in colorectal cancer outcomes, hindering the progress toward our 80% in Every Community goals. In the early stages of the COVID-19 pandemic, leading agencies, such as the Centers for Medicare & Medicaid Services (CMS) and the American Cancer Society, made recommendations to delay all non-urgent procedures. Colonoscopies to detect colorectal cancer have been delayed or cancelled and patient fears about contracting COVID-19 have led to further reductions in screening. This drop has raised concern that COVID-19 related screening delays will lead to missed and advanced stage colorectal cancer diagnoses and to excess deaths from colorectal cancer. Moreover, this burden will likely not be evenly distributed as screening disparities may be exacerbated in communities and populations that are disadvantaged by both old and new challenges in the COVID-19 era.
The colorectal cancer fighting community stands prepared and well-positioned to respond to and overcome the difficult task ahead, and this document offers the latest (as of June 2020) data, research, and clinical guidelines available related to colorectal cancer screening and COVID-19.
Aligning Statements include:
- Despite the challenges we face during the pandemic, colorectal cancer remains a public health priority, and we must provide the public with safe opportunities to prevent and detect colorectal polyps and cancer.
- Colonoscopy remains safe, is a good option for screening, and is quickly reopening around the country, but identifying patients who should receive higher priority for colonoscopic screening is a critical step.
- During a time when availability of elective screening colonoscopy may be limited by the COVID-19 pandemic, colorectal cancer screening can be safely offered through at-home stool-based tests.
- Gaining momentum and reigniting screening activities and public messaging will be highly dependent upon local regulatory requirements, public health priorities, and policy change.
Throughout the pandemic, individuals have options to screen for colorectal cancer. There are many safe, effective, and evidence-based screening tests available, including colonoscopy and non-colonoscopy options (e.g., stool-based tests, stool-DNA tests, and CT colonography). In addition to the information included in the Playbook specific to stool-based testing and colonoscopy, CT colonography also serves as an important option for patients. Learn more in an editorial published in Abdominal Radiology (July 2020), “CT Colonography’s role in the COVID-19 pandemic: a safe(r), socially distanced total colon examination.”
We gratefully acknowledge the contributions of the following individuals and organizations. Thank you to our authors Durado Brooks, Rachel Issaka, Steven Itzkowitz, Michael Sapienza, Ma Somsouk, Richard Wender, Caleb Levell, and Emily Bell. We also extend a special note of gratitude to our committed partners, NCCRT Steering Committee members, and subject matter experts that have contributed to both reviewing and advising on this document, but also for their participation in ongoing discussions aimed at uniting and guiding the colorectal cancer community throughout the COVID-19 pandemic. And finally, we recognize the efforts of the Colorectal Cancer Alliance to provide support, in-kind staff, and continued commitment in coordinating a national response to improving colorectal cancer screening rates during the COVID-19 era.
Explore More

Blue Star Conversation: July 9, 2025
This Blue Star Conversation covered the topic, "2025 Supreme Court Case Update: How Kennedy v. Braidwood Management Could Affect Insurance Coverage for CRC Screening".

Blue Star Conversation: April 15, 2025
This Blue Star Conversation covered, "Modeling the Impact of Colorectal Cancer Screening and Timely Follow-Up Colonoscopy: Recent Data from the Cancer Intervention and Surveillance Modeling Network (CISNET)"

Clinician’s Reference: Stool-Based Tests for Colorectal Cancer Screening
This revised resource is designed to introduce (or reintroduce) clinicians to the value of stool-based testing for colorectal cancer.