Topic or Keyword: Colonoscopy

Tailoring Colorectal Cancer Screening Messaging: A Practical Coalition Guide

Blue Star Conversation – May 17, 2023
NCCRT Learning Center
NCCRT Colonoscopy Needs Calculator
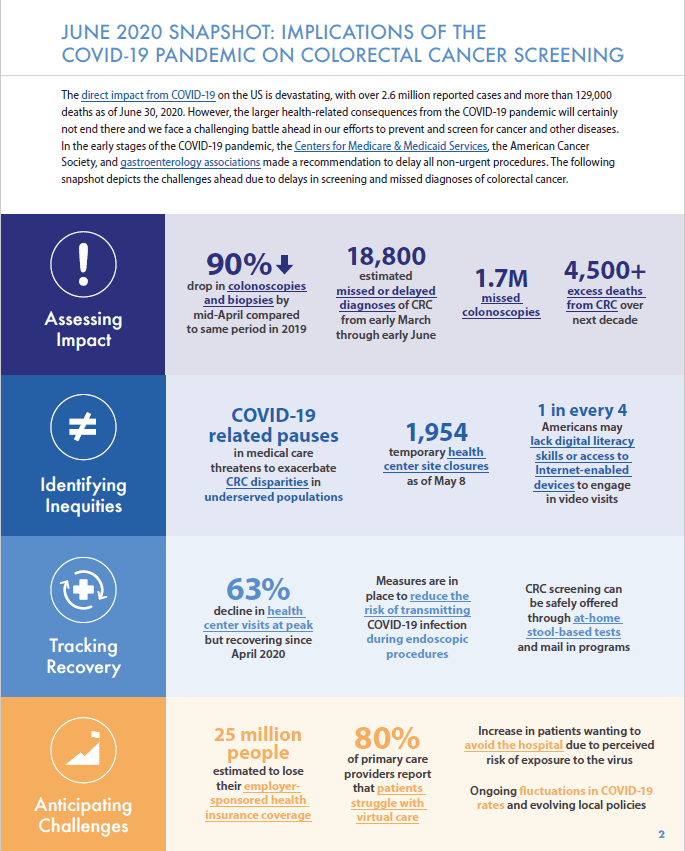
A Playbook for Reigniting Colorectal Cancer Screening as Communities Respond to the COVID-19 Pandemic

Advanced Colorectal Polyp Brief

Colorectal Cancer Screening Handbook for Hospitals and Health Systems

How To Assure Follow Up Colonoscopy For Positive FIT From The Process Side – January 30, 2018
