Topic or Keyword: CT Colonography
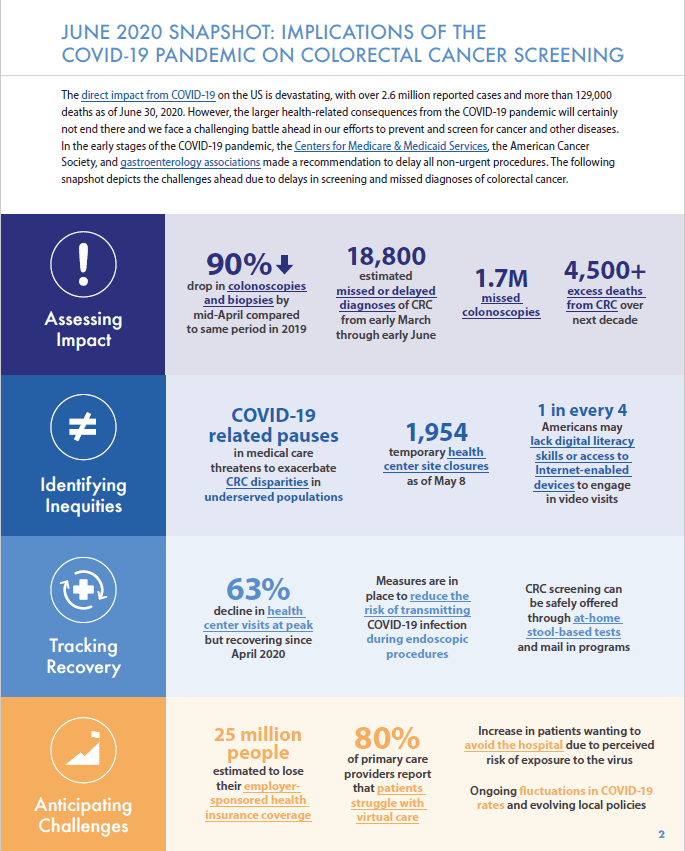
A Playbook for Reigniting Colorectal Cancer Screening as Communities Respond to the COVID-19 Pandemic
My CT Colonography Center Locator Tool

Colorectal Cancer Screening: Recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer

What Can Radiologists Do To Advance 80% By 2018?

Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement
