Media Type: PDF

2022 Messaging Guidebook for Black & African American People: Messages to Motivate for Colorectal Cancer Screening

Webinar: 2022 Messaging Guidebook for Black & African American People: Messages to Motivate for Colorectal Cancer Screening – June 21, 2022

President’s Cancer Panel Cancer Screening Report – February 2022

NCCN Guidelines for Patients: Colorectal Cancer Screening – 2021

How Can Survivors & Families Save More Lives from Colorectal Cancer?

How Can Employers Save More Lives from Colorectal Cancer?
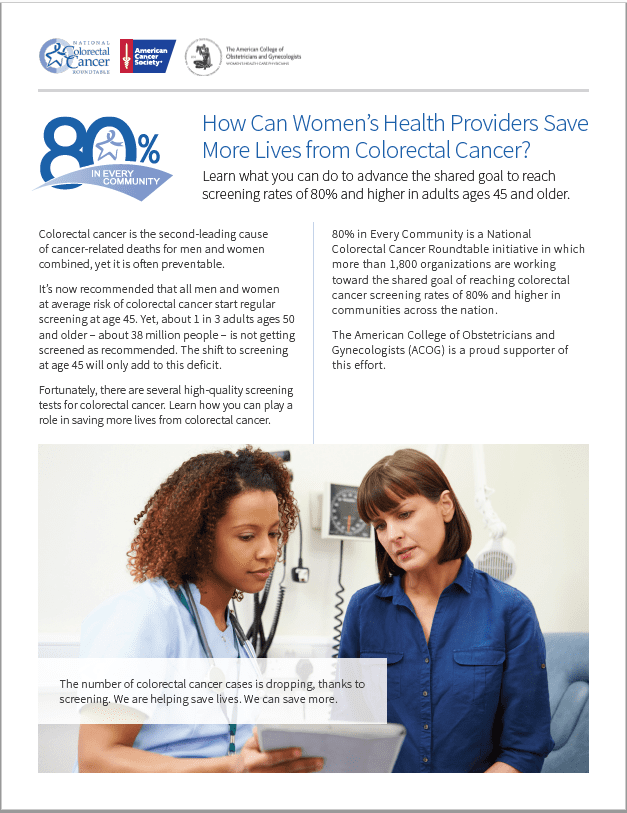
How Can Women’s Health Providers Save More Lives from Colorectal Cancer?

Blue Star Conversation – What Proportion of Early-Onset Colorectal Cancer is Potentially Preventable Based on Family History and Genetics? – March 29, 2022
